Explain with neat sketch the constructional features of ‘Three Way Catalytic Converter’.
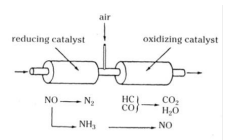
- Three way convertor uses thin coating of platinum, palladium and rhodium over a support metal (generally alumina) & acts on all three major constituents of exhaust gas pollution i. e. hydrocarbons, carbon monoxide & oxides of nitrogen, oxidizing these to water, carbon dioxide & free hydrogen & nitrogen respectively. - It operates in two stages, the first convertor stage uses rhodium to reduce the NO2 in the exhaust into nitrogen & oxygen. In second stage convertor platinum or palladium acts as oxidation catalyst to change HC & CO into harmless water & CO2. - For supplying the oxygen required in the second stage air is fed into the exhaust after the first stage. - Reactions within catalyst produce additional heat that reaches temperature of 900oC, which is required for the catalytic converter to operate at complete efficiency. To safeguard from this high temperature, the catalytic converter is made of stainless steel &special heat shields are also used.
